Recruiting Research Participants
HRPP/IRB Review
The HRPP and IRB are charged by federal regulations, as outlined by the U.S. Department of Health and Human Services (HHS), with ensuring that the selection of participants for research is equitable. They also check to make sure recruitment activities follow Brown’s Policy on the Recruitment of Human Subjects Research Participants.
To meet this obligation, HRPP staff and IRB members evaluate the purpose of the research and the setting in which the research will be conducted. They assess the research to ensure that the recruitment process is appropriate and free from coercion and undue influence, and give particular attention to research involving special or vulnerable populations to guarantee that additional protections are included to protect the rights and welfare of the participants.
- Brown Policy on the Recruitment of Human Subjects Research Participants
- Federal Regulations for the Protection of Human Subjects in Research
- IRB Functions and Operations
Image Review
The HRPP and IRB may review images to ensure they are appropriate for the population and do not create a potential for risk or harm (i.e., undue influence or offensive content).
Modifications
All content changes, including for recruitment materials, require HRPP/IRB review through a modification request in the online submission platform (Huron). All changes must receive HRPP/IRB approval prior to going live on a flyer, email or platform.
Exceptions
Investigators can request an exception to an approved policy during an initial study or modification review. Submissions that include a policy exception request will be reviewed at a convened meeting by the full IRB.
Participant Withdrawal
Enrolling in a research study is voluntary. Every participant is allowed to withdraw from a study at any time during the research. So too, you might need to terminate a study session for reasons that have nothing to do with the research or if the participant is not following the terms of the study.
If a participant withdraws during a study procedure or if you terminate the study session, you must provide the participant with the full compensation (monetary or course credit) for that session, as listed in the compensation section of the consent document.
If the participant withdraws before the study procedure (i.e., cancels appointment or does not appear for the appointment), you do not need to compensate the participant for that session unless the study consent document states otherwise.
Student Participation in Research
By federal law, research participation can only be voluntary and research administrators must minimize the possibility of coercion and undue influence. Researchers should ensure that nonfinancial incentives are not so great that they reduce a person’s voluntariness to consent to participation or their ability to appreciate the potential risks involved.
Whenever research participation is a course requirement, or when extra credit or course credits are offered for participation, students must be informed of nonresearch alternatives involving comparable time and effort to fulfill the course requirements or obtain the credit in order for the possibility of undue influence to be minimized. Students cannot be penalized for refusing to participate in research. It must be clear that choosing not to participate in research will not in any way adversely affect a student’s relationship with Brown or their professors, teaching assistants or peers.
Faculty members are encouraged to work directly with subject pool administrators to ensure that their nonresearch alternative options and research studies are equitable. Examples of nonresearch alternatives that might take roughly the same amount of time and effort as research participation include: reading journal articles or empirical papers and answering questions, writing a one- or two-page paper about comparable research or attending colloquia.
Recruitment Material Requirements
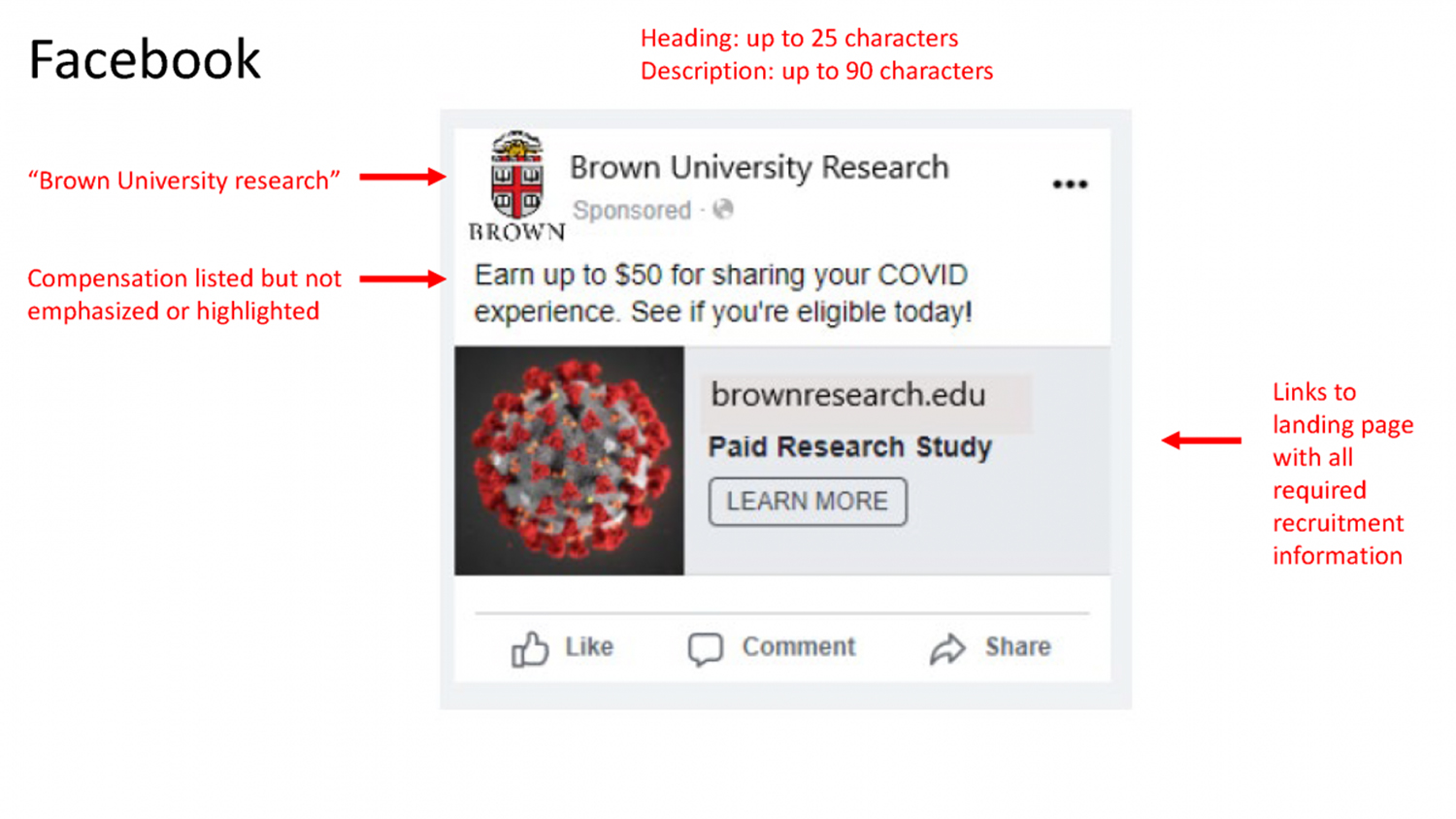
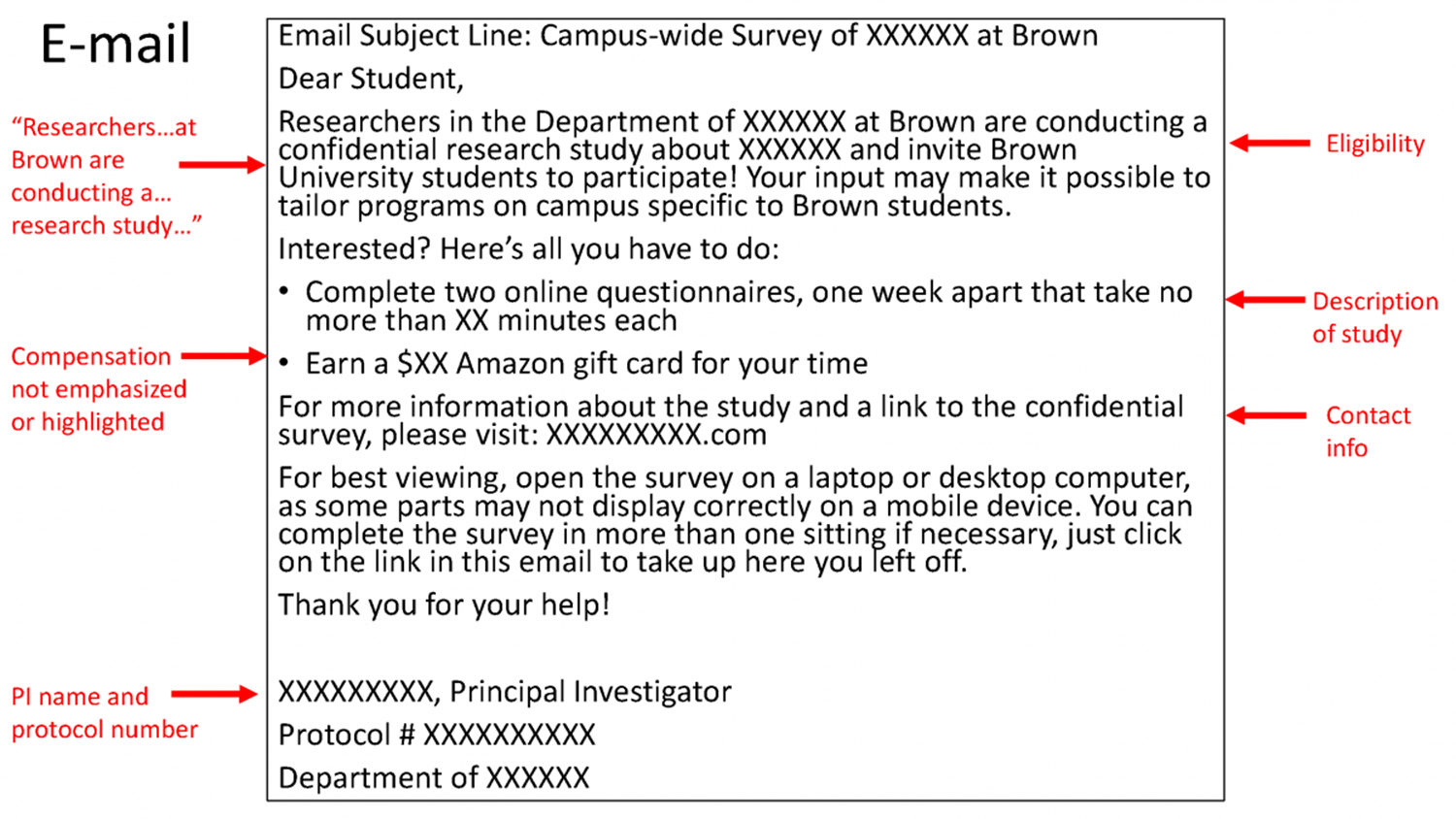
HRPP staff and the IRB review recruitment materials for all research protocols, including but not limited to, scripts, flyers, digital advertisements, video and audio, teaser ads and landing pages. Recruitment materials must contain enough information to give potential participants a sense of the study and the ability to determine whether they may be eligible to participate.
Recruitment is part of participant selection; however, a consent document is not recruitment material and cannot serve as a recruitment landing page. Complete recruitment process and materials must come before the consent process and document.
Using Personal Contact Information
Only Brown University contact information can be used on outward facing materials such as recruitment or consent documents. If you do not have a University phone number, you can reach out to the Office of Information Technology (OIT) to receive one. You can also work with OIT to set up a study-specific Brown University email.
Recruitment Examples
These examples show best practices for advertising research recruitment on a variety of channels; additional information about each of these channels is provided below.
Internet Recruitment
Electronic recruitment materials (social media sites, listservs, recruitment platforms, applications, newsletters, etc.) must comply with Brown recruitment requirements, as well as the policies of the specific location where the material is posted, whichever is more restrictive.
Materials must be clearly identified as recruitment for a voluntary research study and may not be located or posted in any way that could be easily mistaken for, or confused with, employment or paid work. For example, recruitment ads on the Craigslist website must be listed on the Volunteer page and not under Jobs, to avoid the implication that it is employment.
Private listservs or forums may only be used with the permission of the owner or administrator, including private or "invitation only" groups on social media platforms.
Teaser Ads
Teaser ads are used to raise people’s interest by including minimal content. These types of ads are helpful tools for researchers when platforms have content restrictions, such as word count or imagery, and the required recruitment elements cannot be included. These ads must link potential participants to a landing page that includes all required recruitment information. Per Brown’s recruitment policy, teaser ads do not meet the requirements for a complete recruitment process and may not transfer prospective participants directly to a consent process.
Third-Party Recruiting
Third-party recruitment occurs when a principal investigator (PI) asks a personal contact, organization or commercial entity outside of the research team to recruit on behalf of the research team. Recruitment material from a third-party recruiter must clearly reflect that the third-party is not involved in the research study and instruct potential participants to indicate their interest in the study by contacting the research team directly.
Influencers or content creators can be a part of recruitment if they meet the criteria for third-party recruiters and abide by Brown’s recruitment policy.
Third-Party Recruitment Methods
The following are appropriate ways of working with a third party to recruit research participants:
- Have an organization forward an email from the study PI to potential participants
- Have an organization share with potential eligible participants a study flier
- Use a “consent to contact” page to confidentially gather participant consent for the research team to contact them
Unacceptable Third-Party Recruitment Methods
Third-party recruiters may not:
- Collect any research-related information from potential participants used to determine eligibility
- Provide information to a PI about potential participants that would breach the potential participants’ confidentiality or privacy
- Receive compensation for recruitment, unless they are a commercial entity hired to recruit as a service and have no relationship with the potential participants
Snowball Sampling
PIs may ask enrolled participants to recruit prospective participants (i.e., “snowball sampling”). The HRPP team and IRB generally agree that enrolled participants who do not receive rewards or compensation for referrals are unlikely to induce bias, or feel undue influence or coercion. Appropriate measures must be taken by the PI and enrolled participants to ensure that the confidentiality of prospective participants is not violated during this process.
Compensation for Referrals
Payment, compensation, reward or bonuses in exchange for referrals of potential participants from others (known as “finder’s fees”) is not permitted unless the recruiter is a commercial entity hired to recruit as a service and has no relationship with the potential participants. Compensation of this kind may place prospective and enrolled participants at risk of coercion or undue influence, or cause inequitable selection of participants.
Recruiting via Today@Brown
Solicitations for human subjects research recruitment through Brown’s daily email digest Today@Brown are permitted for Brown research faculty if the study has ever been supported by sponsored funding and has Brown HRPP/IRB approval to use this recruitment procedure. PIs whose association with their sponsor ended within the last two years (or within the last two years of their no-cost extension), may continue to use Today@Brown to recruit for their previously funded study. To do this, PIs must inform the IRB/HRPP that their intended advertisement on Today@Brown is consistent with the research that was funded by the sponsor.
Research under a reliance agreement (i.e., IRB Authorization Agreements [IAA]) in which Brown relinquishes IRB oversight is not eligible for recruitment using Today@Brown.
Today@Brown Requirements
When requesting to use Today@Brown for recruitment, researchers must adhere to the approval criteria laid out in Brown’s recruitment policy. The recruitment material must include the subject line that will be used in the Today@Brown posting as well as the full text to be used in the advertisement. The subject line may not feature or highlight any study compensation.
After HRPP/IRB approval, researchers who wish to use Today@Brown must:
- obtain a letter affirming that the HRPP team or IRB have approved the use of Today@Brown as a recruitment tool for a specific study
- email the approval letter to today@brown.edu on the same day as their submission to Today@Brown; and
- include the following copy in the Today@Brown submission message: “Use of Today@Brown for recruiting participants has been approved by Brown’s Human Research Protection Program.”
Crowdsourcing
Crowdsourcing is obtaining information by enlisting a large number of people, either paid or unpaid, typically via digital platforms. Crowdsourcing is frequently used to recruit for human subjects research studies. All recruitment materials used for crowdsourcing must follow Brown’s recruitment policy. If crowdsourcing does not allow for HRPP/IRB review and approval of recruitment material it may not be used for human subjects research studies at Brown.
Approved Software
OIT is responsible for determining which software and services are available for Brown human subjects research. For a list of available applications, view the OIT Software Catalog. Investigators are strongly encouraged to direct prospective participants to a Brown data collection platform, such as Qualtrics, in order to consent participants and conduct human subjects research activities.
If it is an important part of your research design to collect participant information from a crowdsourcing platform that OIT has not yet vetted, the platform will require ancillary review by OIT. This review will not delay HRPP/IRB review or approval of your research, but it may delay implementation of your HRPP/IRB-approved procedures.
Crowdsourcing and Compensation
The Controller’s Office is responsible for ensuring the proper stewardship of the University’s financial resources and determines which forms of compensation comply with federal and state laws, and with University policy. Only these forms of compensation are available to you for human subjects research.
Compensation for Research Participants
Confidentiality in Crowdsourcing
Concerns about privacy and confidentiality vary depending on the type of crowdsourcing platform you use and the research you conduct. Required consent language specific to HRPP/IRB-approved crowdsourcing should be added to the confidentiality section of your consent document.
Research Subject Pools
A research subject pool is a common recruitment method for researchers, and many Brown departments and labs have chosen to set up subject pools. All human subjects research studies must have HRPP/IRB approval to use subject pools for recruitment.
Anyone can join a subject pool. By joining a “subject pool,” a person is showing their interest to participate in research. Joining the subject pool does not mean they have consented to participate in a research study, because they have not yet been provided with sufficient information concerning the exact study in which they might participate. Until they go through a consent process, a member of a subject pool is not a participant in a research study. All subject pool members are free to decline participation in any research study at any time.
Research under an IAA in which Brown relinquishes IRB oversight cannot use subject pools for recruitment.
Recruiting for Subject Pools
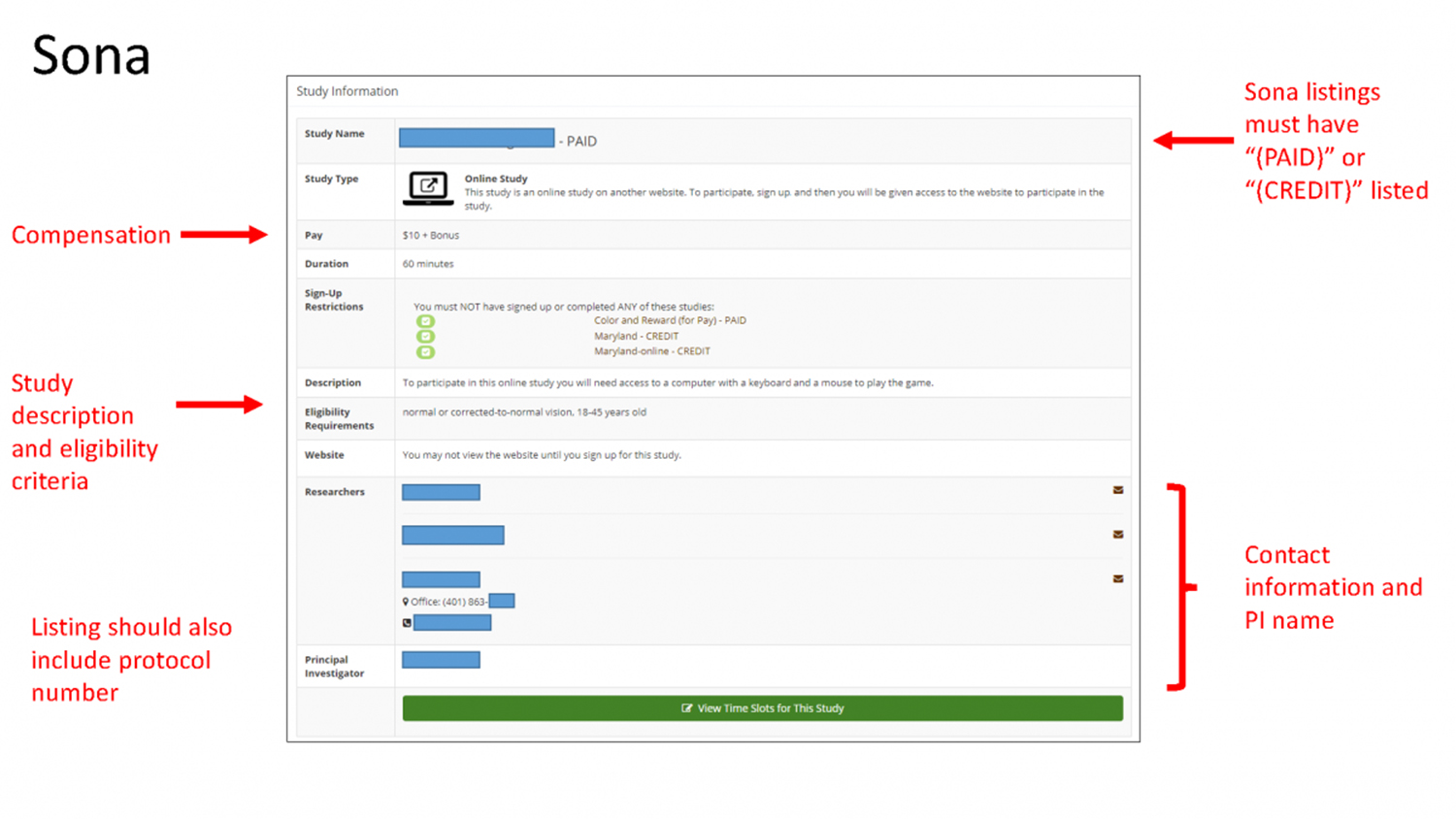
All recruitment materials must meet the requirements for approval as described in Brown’s recruitment policy and include the protocol number and first name (at a minimum) of the PI or a member of the research team. Researchers using the Sona Systems recruitment platform also must include “(PAID)” OR “(CREDIT)” after the study title.
As long as you are seeking volunteers to join a subject pool and not prospective participants for research studies, you do not need HRPP/IRB approval to use Today@Brown.
Subject Pool Compensation
Compensation for study participation in the subject pool may be in the form of cash, gift cards or Brown course credit. Although you can use a variety of payment options through a subject pool, you may not combine them. You must decide whether compensation will be monetary or course credit, and this must be clearly reflected in your recruitment material.
Research Involving Deception
You may use a subject pool if the research involves deception; however, you should use a debriefing process if appropriate for your study design. This debriefing process should occur within a reasonable amount of time after the participant has completed the study procedures or when the study is over. During this process, participants must be told about the nature of the deception and, if the data is identifiable, you should ask permission for continued use of the data.
Unacceptable Recruitment Methods
HRPP/IRB may at times decide that certain recruitment methods or services are not allowed for human subjects research studies.
Currently, HRPP/IRB does not allow researchers to use the Lucid survey sampling service for recruitment. Lucid creates marketing materials as part of its service; however, the company does not share its material with the customer (the PI and Brown University) for review or approval before use with prospective participants. Because all recruitment materials require HRPP/IRB approval prior to their use, Lucid may not be used for recruitment of prospective participants in human subjects research studies at Brown.
Referral Compensation
Payment, compensation, reward or bonuses in exchange for referrals of potential participants from others (known as “finder’s fees”) is not permitted unless the recruiter is a commercial entity hired to recruit as a service and has no relationship with the potential participants.
Compensation of this kind may place prospective and enrolled participants at risk of coercion or undue influence, or cause inequitable selection of participants.
Other Participants in Research
Compensation
Compensation for Research Participants
Compensating research participants is a way to thank them for contributing their time and recognize their willingness to take on some of the research-related risks of study activities. As compensation is an expression of gratitude by researchers for their participants, it is not a benefit of participation, and it must be fair and equitable. However, offering compensation is not required or expected.