Brown University is committed to the conduct of responsible research. The Human Research Protection Program (HRPP) team and others across the University encourage you, as a potential research participant, to review the information provided to you and discuss it with people you trust before committing to participation in a research study.
Information for Research Participants
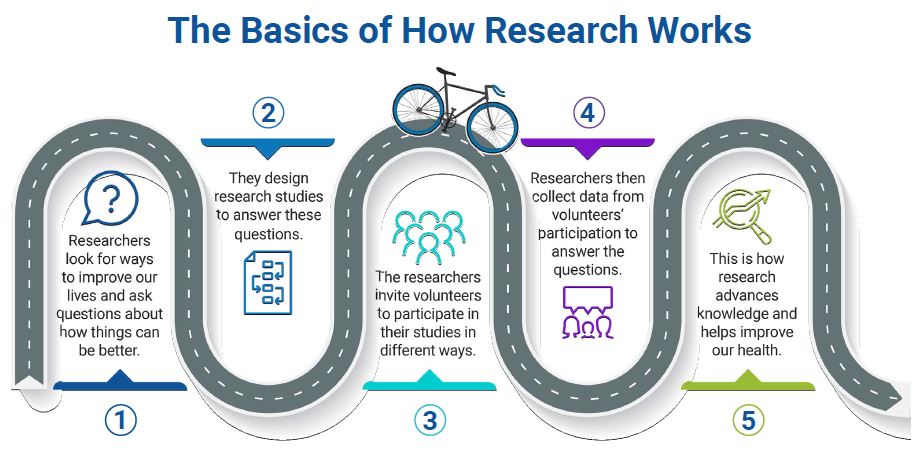
The Basics of How Research Works
- Researchers look for ways to improve our lives and ask questions about how things can be better.
- They design research studies to answer these questions.
- The researchers invite volunteers to participate in their studies in different ways.
- Researchers then collect data from volunteers' participation to answer the questions.
- This is how research advances knowledge and helps improve our health.
What Is a Research Study?
A research study is an organized activity designed to learn more about a problem or answer questions. Many different kinds of studies are conducted. For example, a study may test if a product, such as a drug or equipment, is safe and effective. A study may be done to find out what education practices work best. A study may use a survey or an interview to understand needs, problems, or feelings people have about an important topic. A study may be done to determine the best way to treat or prevent an illness.
Why Volunteer for a Study?
There are many reasons to take part in research. You may want to help scientists find out more about how the human body and mind work, help other people, help find a cure for an illness or have many other reasons for wanting to participate in a study.
Consider the Benefits
There may or may not be a direct benefit to you if you take part in a research study. You may get better as a result of your taking part in the study, you may stay the same, or you may even get worse. No one can predict the outcome of a research study or how it might affect you. The study may not help you personally, but your taking part may result in information that helps others in the future.
Understand the Risks
Sometimes research procedures may cause discomfort and side effects. The questions being asked could make you uncomfortable. The risks and side effects of the research may not be known completely when you start the study. The research staff will discuss with you known possible risks so you can decide if you want to volunteer. If you do volunteer, the research staff will tell you about any new risks that they learn about during the study for as long as you take part in the study.
Know Your Rights as a Study Participant
If you decide to take part in a research study, you do so as a volunteer. That means you decide whether or not you will take part. If you choose to do so, you have many important rights.
Consider these important points when considering whether to participate in a study:
- Your decision will not affect your relationship with the organization conducting the research.
- If anyone asks you to take part in a research study, you have the right to say “no.”
- You need to weigh both the risks of the study and the benefits.
- It may be helpful to talk with family, friends or other people you trust as you decide whether to participate.
- If you decide to take part in a research study, you can change your mind and stop or leave the study any time without anyone holding it against you.
Ask Questions
Before you decide to volunteer to take part in a research study, you need to know as much as possible about the research study. If there are any issues that concern you, be sure to ask questions. You might want to write your questions down in advance or print out this page to take with you. The following is a list of sample questions. Not every question will apply to every study. Remember, if you do not understand the answer to one of your questions, ask the question again and ask the person to explain the answer in a way you can understand it. If you forget the answers to the questions during the study, just ask them again.
- Who is doing this study and what question might it answer?
- Will this research help in understanding my condition? If so, how?
- What tests or procedures will be done?
- Will I have to make extra trips?
- What could happen to me, good and bad, if I take part in the study?
- How long will this study last?
- What will happen to any specimens that I give?
- Who has reviewed and approved this study?
- Could I get worse during the study? What will happen if I do?
- What other options or choices do I have if I decide not to take part?
- Will I be charged anything or paid anything to be in this study?
- If I decide to take part in this study, how will it affect my daily life?
- What will happen to me at the end of the study?
- Will I be told the results of the study?
- Who will find out that I am taking part in this study?
- How do I stop taking part in this study if I change my mind?
- Whom do I contact for questions and information about the study?
- Whom do I contact for questions and information about the study?
- What will happen to my private information?
Informed Consent
Informed consent is the process of learning the key facts about a research study before you decide whether or not to volunteer. Your agreement to volunteer should be based upon a clear understanding of what will take place in the study and how it might affect you. Informed consent begins when the research staff explains the facts to you about the research study. The research staff will assist you with the informed consent document that goes over these facts so you can decide whether or not you want to take part in the study. These facts include details about the study, procedures you may receive, the benefits and risks that could result, and your rights as a research volunteer. Please note that the informed consent document is not a legal document.
You should take your time when you read the consent form. If you have any questions, ask the research staff. If you don’t understand something, ask them to explain it to you so you do understand. If English isn't your native tongue, ask for an interpreter to be present when you are discussing the study with the research staff. The written and verbal informed consent information will be given to you in a language that you know. You can take the information home with you and discuss it with your family, friends, a health care provider, or others before you decide whether or not to take part in the study.
If you decide to take part in the study, you may be asked to sign the informed consent form. However, the informed consent process is more than just signing a piece of paper. It is a process that goes on throughout the study. During the course of the study, you may be told of new findings, benefits or risks. At that time, you can decide whether or not to continue taking part in the study. You may change your mind and leave the study before it starts or leave at any time during the study or the follow-up period.
Who Will See Your Records?
Like your medical record, the information in your research record will be confidential. Information will be given only to the researchers who carry out the study or to those who make sure that the study is safe and carried out the way it was planned. The groups of individuals who might look at your records are the research staff, the Institutional Review Board (IRB), the company or group funding the study and various government oversight agencies. It is important for these groups to be able to look at your records so they can ensure that the study is conducted using acceptable research practices.
The Role of the IRB
The IRB is a group of people such as scientists, nonscientists and people from the local community who ensure that human research is ethical. The IRB serves to protect your rights and your welfare before and during the research study. For example, the IRB makes sure that any risks in the research study are as small as possible.
The IRB does not make a decision for you. The IRB decides, when approving research studies, that it is reasonable to ask people whether they want to be involved in it. The IRB also reviews each study while it is going on to make sure volunteers are protected.
Submitting Questions, Concerns or Complaints
If you have any questions, concerns or complaints about research you can report your concerns to Brown’s HRPP team. HRPP staff members will accept anonymous and collect calls and provide an interpreter if requested.
- Phone: 401-863-3050
- Email: irb@Brown.edu
Submitting Complaints and Concerns
Research participants, their family members and others may have complaints, concerns or questions about a Brown research study and are encouraged to submit these concerns to the Human Research Protection Program (HRPP) confidentially or anonymously.